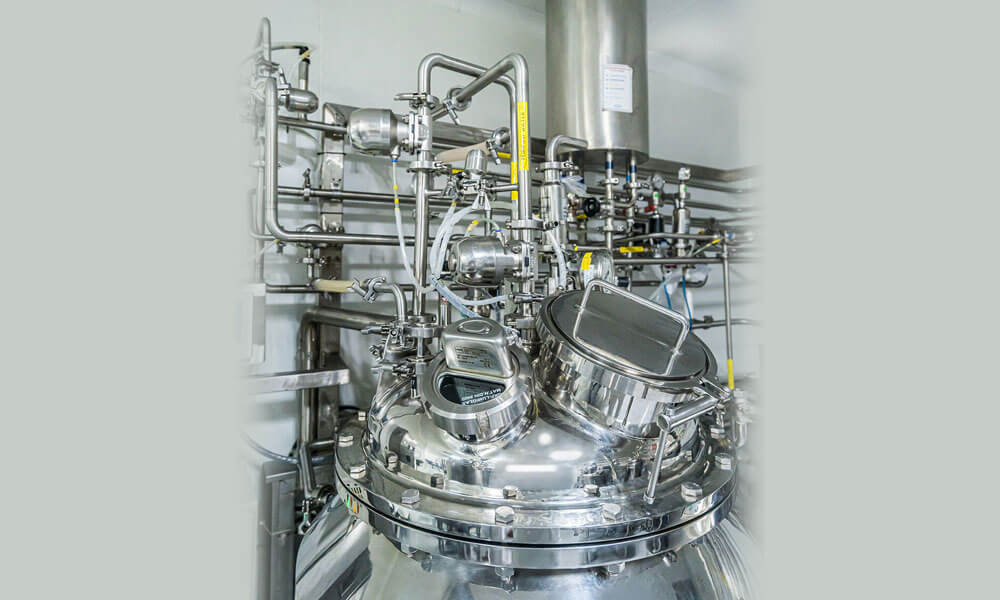
Cleaning-In-Place (CIP) & Sterilization-In-Place (SIP) are systems designed for automatic cleaning & disinfecting. We design, develop, manufacture, supply and install CIP & SIP for sanitization & sterilization. The system is custom made, modular, skidded in automated or semi-automated Models as per the required time cycle for cleaning & sterilization of large fixed multi-tank system.
With the pharmaceutical & biopharma industry getting matured day by day with constant check by regulatory bodies and the trained of designing multiproduct manufacturing facility requires an articulated approach to ensure cleaning & Sterility of the system.
All systems are tested at our dedicated FAT testing area that are fully equipped with all required utilities. We ensure all instruments should work fine under the designed conditions. Our QA team ensures complete documentation that are required before and during the FAT/SAT.


















Copyright © IUS Equipment Pvt. Ltd. All Rights Reserved